Technological developments, such as precision medicine, next-generation sequencing (NGS), and long-read sequencing have made significant advances in science and technology and simultaneously opened avenues for public healthcare. As the next frontier of medicine, the global molecular diagnostics market showcases enormous potential to completely revolutionize patient care and overall wellness in general.
The importance of diagnostic tests throughout the entire procedure (i.e., prevention, detection, diagnosis, treatment, and successful management of health conditions) of treating an individual for a particular disease is as consequential as the treatment itself. It has been subjected to extensive research for further refinement of the same.
Our healthcare experts have found the molecular diagnostics market to be one of the stable markets, and the global market is predicted to grow from $10,914.6 million in 2020 to $24,228.0 million in 2031 and is expected to grow with a CAGR of 7.38% during the forecast period 2021-2031.
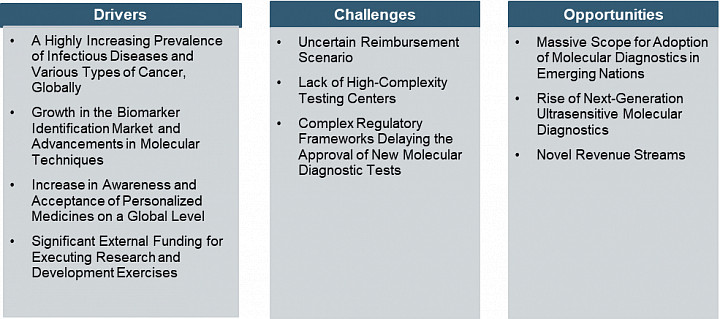
Factors fueling the growth of the market include a highly increasing prevalence of infectious diseases and various types of cancer, an increase in awareness and acceptance of personalized medicines on a global level, and significant external funding for executing research and development exercises. Despite rapid advanced industry growth, several key issues need to be addressed to facilitate future growth. Uncertain reimbursement scenarios, lack of high-complexity testing centers, and complex regulatory frameworks delaying the approval of new molecular diagnostic tests are hampering the market growth. Further, some of the opportunities, such as massive scope for the adoption of molecular diagnostics in emerging nations and the rise of next-generation ultrasensitive molecular diagnostics and novel revenue streams, provide growth to the market.
Market Segmentation
• Products (Kits and Consumables, Systems, Software and Other Products)
• Testing Location (Laboratory Testing and Point-of-Care (POC) Testing)
• Application (Core Molecular Diagnostics, Reproductive Genetics, Companion Diagnostics (CDx), Liquid Biopsy, and Others)
• Technology (Polymerase Chain Reaction (PCR), Next-Generation Sequencing, Isothermal Nucleic Acid Amplification Technology (INAAT), Microarray, In-situ Hybridization (ISH), Immunohistochemistry (IHC) and Other Technologies)
• End User (Hospitals, Diagnostics Centers, Out-Patient Clinics/General Practitioners, Research Laboratories, and Other End Users)
Regional Segmentation
• North America: U.S., Canada
• Europe: Germany, France, Italy, U.K., Spain, and Rest-of- Europe
• Asia-Pacific: Japan, China, India, Australia, Singapore, and Rest-of-Asia-Pacific
• Latin America: Brazil, Mexico, Rest-of-Latin America
• Rest-of-the-World (RoW)
Competitive Landscape
The exponential rise in the prevalence of infectious diseases and various types of cancer globally has created a buzz among companies to invest in advanced technologies such as molecular diagnostics.
Based on region, North America holds the largest share, owing to improved healthcare infrastructure, rise in per capita income, and improvised reimbursement policies in the region. However, the Asia-Pacific and Europe regions are anticipated to grow at the fastest CAGR during the forecast period.
Molecular Diagnostics Market - Market Dynamics
Key Companies Profiled
Abbott, Agilent Technologies, Inc., Becton, Dickinson and Company (BD), bioMérieux SA, Bio-Rad Laboratories, Inc., Danaher, F. Hoffmann-La Roche Ltd, Guardant Health, HTG Molecular Diagnostics, Inc., Illumina, Inc., Invivoscribe, Inc., ICON plc, LungLife AI, Inc., QIAGEN, QuantuMDx Group Ltd., Siemens Healthcare GmbH, Thermo Fisher Scientific Inc.
Key Questions Answered in this Report:
• How is each segment of the market expected to grow during the forecast period 2021-2031, and what is the anticipated revenue to be generated by each segment?
• What are the major market drivers, challenges, and opportunities in the global molecular diagnostics market?
• What are the underlying structures resulting in the emerging trends within the global molecular diagnostics market?
• How is each segment of the global molecular diagnostics market expected to grow during the forecast period, and what will be the expected revenue generated by each of the segments by the end of 2031?
• What are the key developmental strategies implemented by the major players to sustain in the competitive market?
• What are the key regulatory implications in developed and developing regions for molecular diagnostics?
• Who are the leading players with significant offerings to the global molecular diagnostics market? What is the current market dominance for each of these leading players?
• What would be the compound growth rate witnessed by the leading players in the market during the forecast period 2021-2031? Which molecular diagnostic product type has the most promising growth?
• What are the key applications in the global molecular diagnostics market? What are the major segments of these applications? What technologies are dominating these application segments?
• What are the major technologies that are employed in the global molecular diagnostics market? Which is the dominating technology?
• Who are the primary end-users of the global molecular diagnostics market? Which is the fastest-growing end-user segment in the global molecular diagnostics market?
• Who are the key manufacturers in the global molecular diagnostics market, and what are their contributions? Also, what is the growth potential of each major molecular diagnostics manufacturer?
• What is the scope of the global molecular diagnostics market in North America, Europe, Asia-Pacific, Latin America, and Rest-of-the-World? Which molecular diagnostics application and end user dominate these regions?
• What are the emerging trends in the global molecular diagnostics market? How are these trends revolutionizing the diagnostic procedure?
• Which technologies are anticipated to break through the current molecular diagnostic regime?
• Which companies are anticipated to be highly disruptive in the future and why?
• What are the regulatory procedures that are required to unify the approval process for emerging molecular diagnostics? How will these enhance the reimbursement scenario?
• What are the gaps in regularizing optimum molecular diagnostic adoption in regular healthcare routines? How are these gaps being tackled?
Request Free Sample - https://bisresearch.com/requestsample?id=1209type=download
The scope of the report exclusively covers manufacturing companies that are actively involved in the development and commercialization of molecular diagnostics products, which predominantly include systems, kits and consumables, and software and other products. The study also takes into consideration the various diagnostics application offered by the manufacturers and service providers.
BIS Related Studies
Liquid Biopsy Market - A Global and Regional Analysis

Comments
Post a Comment